(1) Quality assurance responsibility of raw and auxiliary materials
(2) Product quality assurance responsibility in the production link
(3) Product quality assurance responsibility before storage after inspection
(4) Product quality assurance responsibility after storage
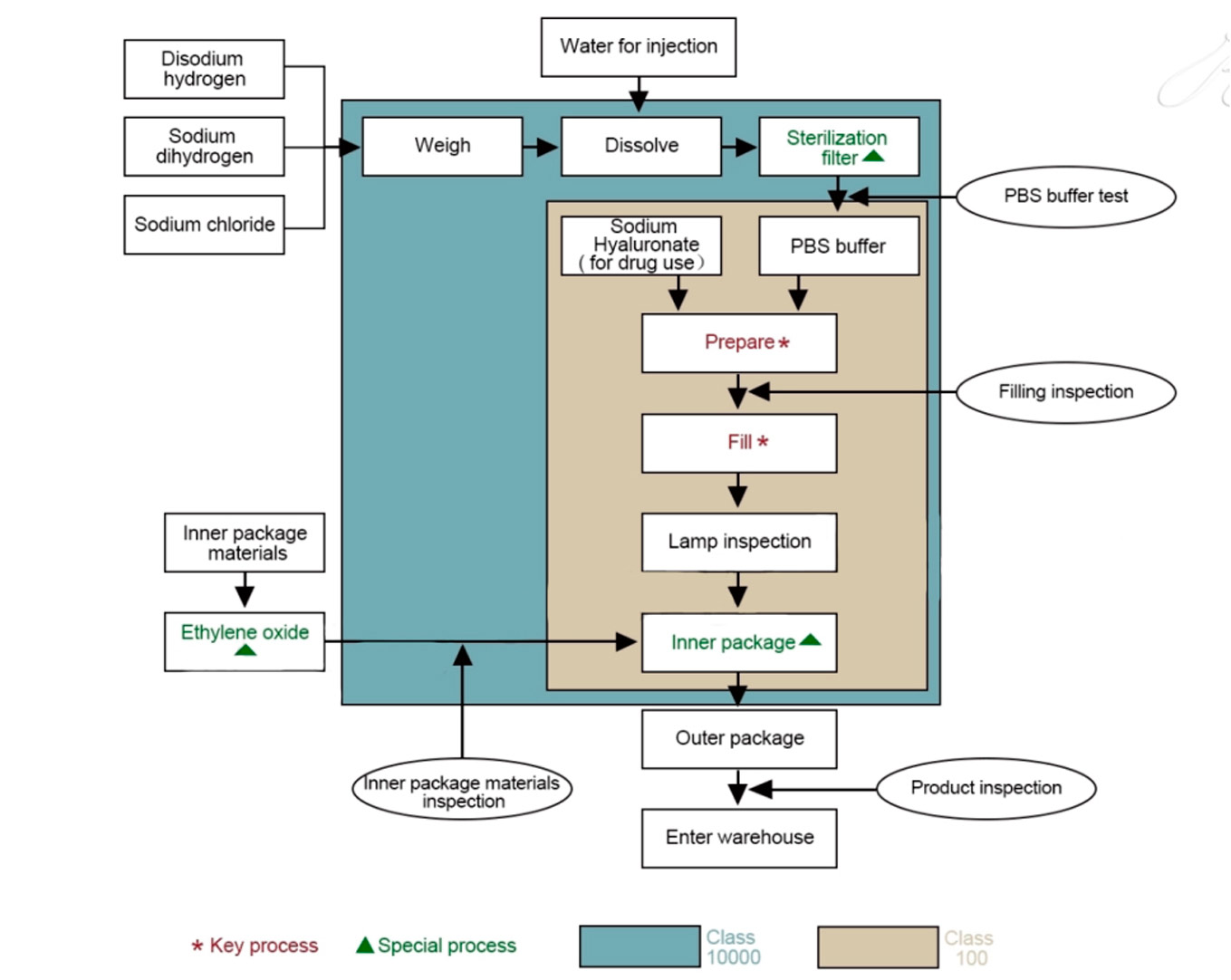
When the production department starts up and changes the specifications and varieties, the online quality inspector of the quality Department will conduct the first inspection. Only after the first inspection is qualified can the production continue. In addition, this inspector is responsible for inspection and certification in the production process, and truly supervises and records the quality of products, and timely reports to higher-level leaders if any abnormal phenomenon occurs. The disputed products and products that cannot be judged quickly should be put in the waiting area for storage, and the non-conforming product declaration form should be issued. The production department shall organize relevant functional departments to review and form the form
The quality department laboratory inspects the performance of the assembled qualified products according to the relevant inspection standards. In production
Field sampling, the tester must be on the spot on the product quantity, size, identification for proofreading, confirm no error before sampling, if there is any doubt, should be timely to the on-duty online quality inspector, quality problems found in the process of performance testing should be reflected to the superior leadership in a timely manner, and according to the standard reinspection, if still unqualified, then timely to the production department
(including the Supply Department if necessary) issue the nonconforming product notice, and the Quality Department shall organize related departments to divide
The nonconforming products shall be isolated by the finished product warehouse.
We strictly control every process of product production, strictly test product quality, to ensure that all products delivered meet market regulatory standards, products have been issued by the UK BSI ISO13485 quality system certification, the EU CE certification.